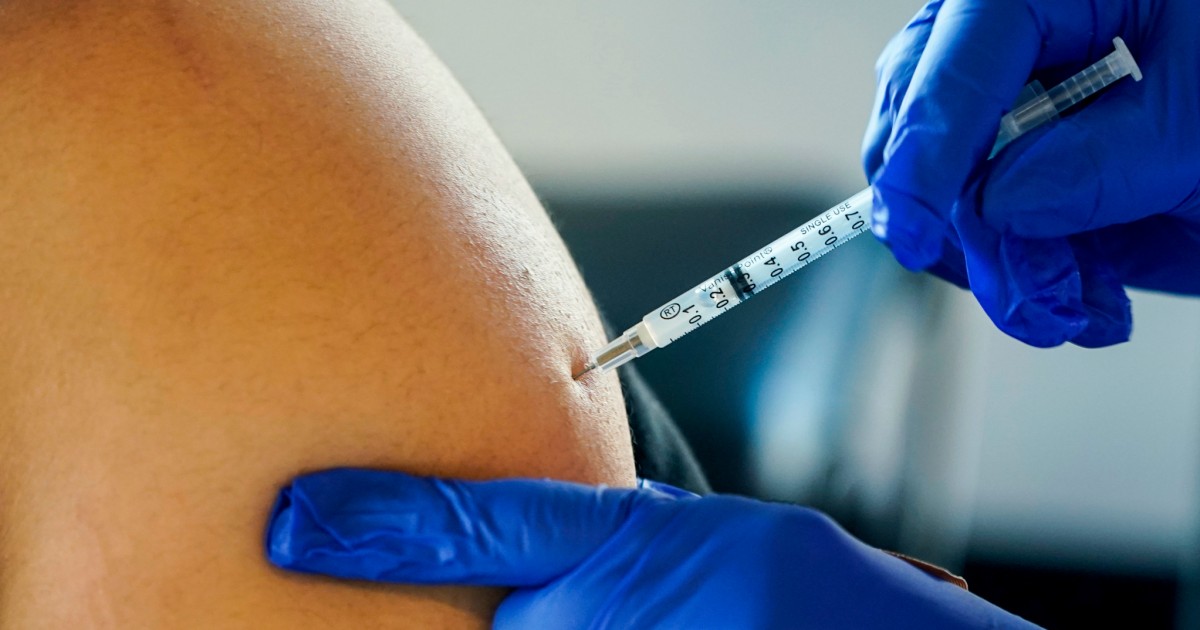
 Pfizer asks Food and drug administration to authorize current booster shot
[ad_1]
Pfizer asks Food and drug administration to authorize current booster shot
[ad_1]
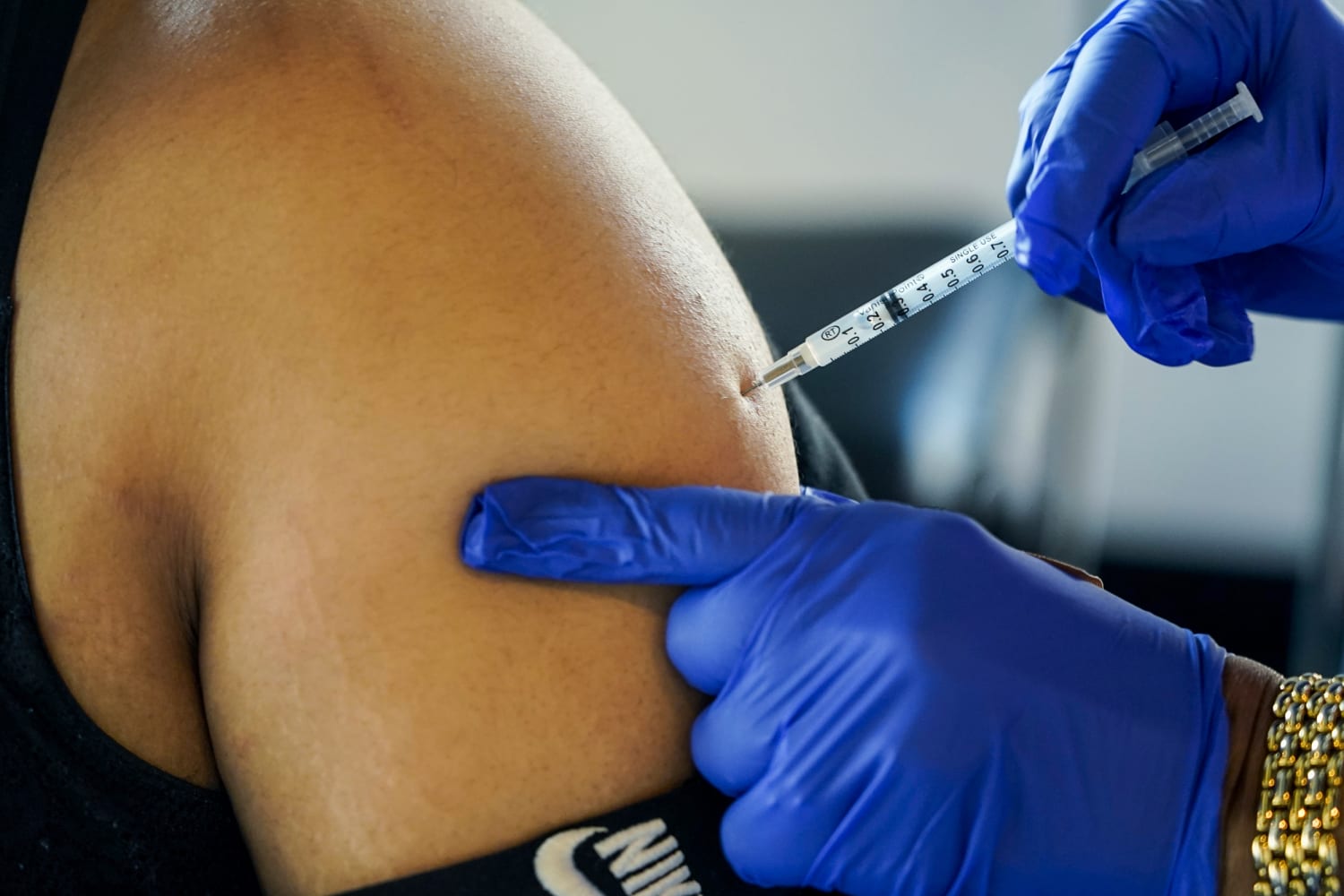
Pfizer-BioNTech questioned the Food stuff and Drug Administration to authorize an up-to-date edition of its Covid booster created to target the BA.5 omicron subvariant, the drugmaker declared in a launch Monday.
Pfizer’s release was sparse on new facts about the efficacy of the booster, but the enterprise claimed scientific studies in animals observed that the vaccine produced an immune reaction versus several variations of omicron, which include BA.4 and BA.5. It is organizing to commence clinical trials in people today this thirty day period, the assertion claimed.
The request, covering people today ages 12 and up, will now be deemed by the Fda, which will assessment the details and is predicted to grant unexpected emergency use authorization for the age group someday in September.
Comprehensive coverage of the Covid-19 pandemic
If authorized, the enterprise reported it is geared up to right away begin distribution of the photographs.
Bill Hanage, a Harvard epidemiologist, praised the "remarkably brief" turnaround time for the new model of the vaccine, declaring it ordinarily usually takes a long time for photographs to be produced and distributed. BA.5 only commenced spreading through the U.S. in early June.
The new vaccine will be "important," especially for individuals vulnerable to extreme infections, such as the elderly.
The current photographs are expected to be rolled out as element of a drop booster marketing campaign ahead of a probable winter surge in Covid cases, White Household Covid coordinator Dr. Ashish Jha said last week.
Pfizer’s so-identified as bivalent booster targets the omicron subvariants BA.4 and BA.5, as effectively as the original pressure of the virus, in a solitary shot. BA.5 accounts for just about 90% of new Covid cases in the United States, according to the Facilities for Sickness Regulate and Prevention.
In June, the firm unveiled clinical demo details on a diverse version of a bivalent shot, one particular that focused the first omicron variant. That booster was shown to be safe and produced an immune response.
The Food and drug administration is permitting Pfizer to post a lot less info on the shot than it has for past Covid vaccines. The agency produced steering final year that mentioned modified vaccines that goal new, emerging strains of the coronavirus could be approved with out the need for prolonged scientific trials.
Jesse Goodman of Georgetown University, a former Food and drug administration vaccine main, explained it "helps make perception" to update the vaccines to focus on new variants circulating.
How productive Pfizer's new bivalent vaccine is in contrast to the present vaccines remains unclear. Goodman said he specifically would like to see how nicely the vaccines carry out from bacterial infections.
It is "possible" the immune responses in individuals will be related to all those observed in the animal studies, he said, but "it is not known until human facts are out there."
If the new model of the vaccine provides much better protection in opposition to infection and serious disease, it could mitigate foreseeable future Covid waves and potentially “take stress off stretched healthcare units,” claimed Dr. Isaac Bogoch, an infectious ailment specialist at the University of Toronto.
Nonetheless, he famous that the lack of data on the bivalent vaccine could make people today a lot more hesitant to get the shot in the near expression, slowing vaccine uptake.
"If you want very good vaccine uptake and you want general public buy-in and public belief, you must have audio information to drive it," he said.
Moderna is also making ready to ask the Fda to authorize its bivalent vaccine that targets BA.4 and BA.5 as well as the unique pressure.
Monday's announcement will come as the current booster photographs, from Pfizer and Moderna, have been a very poor match for omicron and its growing loved ones of subvariants. The shots, which concentrate on the initial coronavirus strain recognized in Wuhan, China in late 2019, even now offer you strong protection against severe ailment.
Follow VFAB Well being on Twitter & Facebook.
[ad_2]




0 comments:
Post a Comment